Is it safe to drink purified water? “Is the most crucial question for health conscious consumers when choosing bottled water or home water filtration systems. Pure water is known for its excellent cleanliness, but discussions about its long-term health effects have never stopped.

Core conclusion:Is it safe to drink purified water?
As a professional in the field of water quality analysis, my answer is: short-term consumption of purified water is safe, but long-term use as the only source of drinking water may pose health risks.
Purified water effectively removes bacteria, viruses, chemical pollutants, and heavy metals from water through processes such as reverse osmosis, distillation, or deionization, which is its core value.
However, this process is also like a double-edged sword, removing harmful substances while also taking away natural minerals that are beneficial to the human body, such as calcium, magnesium, potassium, etc.
Therefore, the “safety” of purified water needs to be understood from two dimensions: short-term no pollution risk, but long-term potential risks of mineral deficiency. For most healthy adults, moderate consumption of purified water is not a big problem, but it should not be seen as the only, long-term drinking water choice.
What is pure water?
Simply put, purified water is high-purity water obtained by removing the vast majority of impurities and minerals from natural water through specific physical and chemical processes.
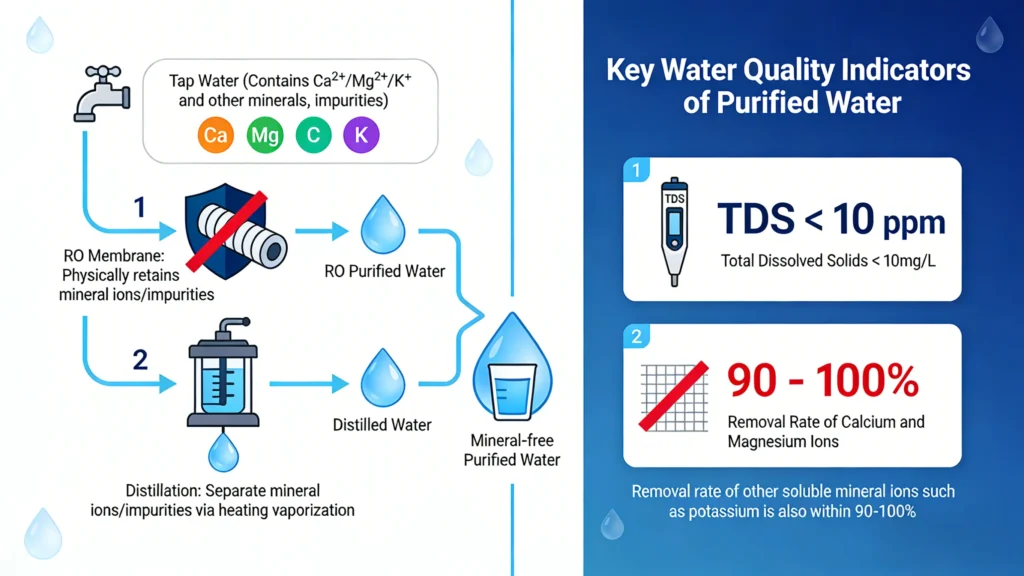
Purified water is not a natural water source, but an artificial state of water quality. Its core indicator is extremely low Total Dissolved Solids (TDS) content. According to strict pharmacopoeias or industry standards, the TDS value of purified water is usually required to be<10 ppm (parts per million), which means that almost only pure water molecules (H ₂ O) remain in the water.

The main characteristics of purified water
| Feature | Purified Water | Natural Mineral Water | Mineral-Added Water |
|---|---|---|---|
| Core Definition | Water highly purified to remove nearly all substances, including minerals and contaminants. | Water from a protected underground source with a stable, natural mineral composition. | Purified water with minerals added back according to a specific formula. |
| Mineral Content | Extremely Low (TDS <10 ppm). Lacks beneficial minerals like calcium and magnesium. | Naturally present & stable. Contains minerals and trace elements from its source. | Artificially added & variable. Minerals are added for taste or health claims. |
| Source & Production | Made from any source water (e.g., tap) via purification processes like Reverse Osmosis (RO) or distillation. | Sourced from a specific, protected spring or well. Usually undergoes minimal treatment. | Starts as purified water; minerals are added in a controlled, industrial process. |
| Primary Goal | To achieve “chemical purity” by removing dissolved solids and contaminants. | To preserve and deliver the natural composition from the source. | To create a consistent-tasting product with a standardized mineral profile. |
| Taste Profile | Neutral, flat, or “soft” due to the absence of minerals that affect flavor. | Unique and varied, directly influenced by its natural mineral content (e.g., earthy, crisp). | Designed and consistent. The taste is engineered through the mineral blend. |
Methods for purifying water
The term “purified” is a specific, regulated standard. According to pharmacopeial guidelines, purified water must contain no more than 10 parts per million (ppm) of Total Dissolved Solids (TDS).
This is achieved not by finding a magical source, but by aggressively processing ordinary water.
- Reverse Osmosis (RO): This is the most prevalent way. Under pressure, water is pushed across a semi-permeable membrane that stops up to 99% of dissolved salts, organics, and microbes. It works quite well, but it uses a lot of water.
- Distillation: Boiling water turns it into vapor, which leaves behind impurities. The vapor is subsequently condensed back into liquid. It gets rid of minerals, bacteria, and most compounds that boil at a greater temperature than water.
- Deionization (DI): Water goes through ion-exchange resins that take out mineral ions (salts), making water that is very pure and is widely used in labs.
The core advantages and risks of purified water
Core Advantages
- Reverse osmosis and distillation make water almost pollution-free by getting rid of germs, viruses, heavy metals (including lead and mercury), and chemical pollutants (like pesticide residues).
- Taste and stability: When minerals and other impurities are taken out, the water tastes fresh and neutral, and the minerals don’t change the taste.
- Filtered water is a very important temporary safety measure in places where the water supply is very dirty or where chemotherapy patients have weak immune systems.
Main potential risks
- Lack of important minerals: Removing toxic compounds also removes useful minerals including calcium, magnesium, and potassium. Pure water and an imbalanced diet may reduce mineral intake over time.
- After eliminating alkaline minerals, its pH may be slightly acidic. Long-term and high consumption may cause enamel erosion.
- The “blank carrier” effect: As a highly pure solvent, it may precipitate trace contaminants from aging pipelines or poor plastic containers during storage or pipeline movement slightly more than mineral water.
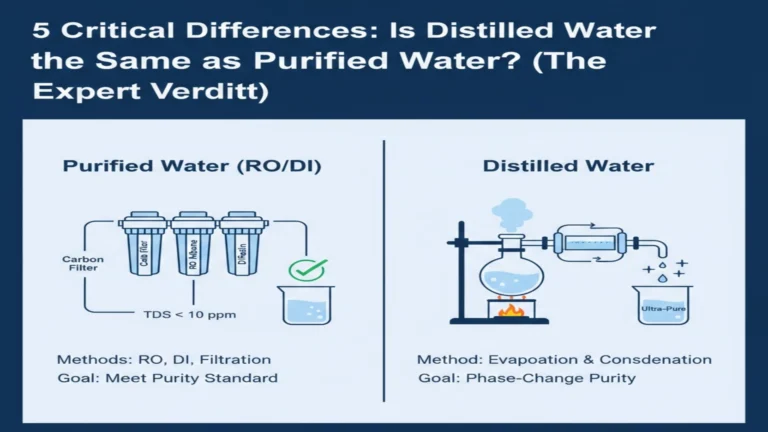
Purified Water vs. Distilled Water
Two typical varieties of “pure water” are distilled water and purified water. The main distinctions between them are how they are made and how pure they are at the end.To put it simply, all distilled water is pure water, but not all pure water is distilled water.
- Purified water is a comprehensive term that includes water that has been cleaned in many different ways, like reverse osmosis, filtration, and so on. The mineral composition is determined by the specific procedure.
- Distilled water is water that has been created by boiling and condensing it. This procedure can almost completely remove minerals and electrolytes from water, making it the most “empty” water because it has very few chemicals.
The pH number shows one of the most important scientific differences. In theory, distilled water should be neutral, but since it is so pure, it quickly takes in carbon dioxide from the air and turns it into carbonic acid. You have confirmed in your experimental blog that this often makes the pH value of distilled water weakly acidic (about 5.8), whereas the pH value of purified water is closer to neutral.
- People often drink and cook with purified water since it is safe.
- Distilled water is usually used in professional settings like labs, medical equipment, and car batteries where there can’t be any scale or ion interference.

Final Summary
Purified water is safe in removing pollutants, but there may be limitations in providing comprehensive health support. Its safety is reflected in its ability to effectively filter out harmful substances such as bacteria and heavy metals; Its limitations stem from the fact that the deep purification process also removes natural minerals that are beneficial to the human body. Therefore, it can be a part of your drinking water choices, but it is not recommended as the only long-term source of drinking water.
FAQ
Related Products
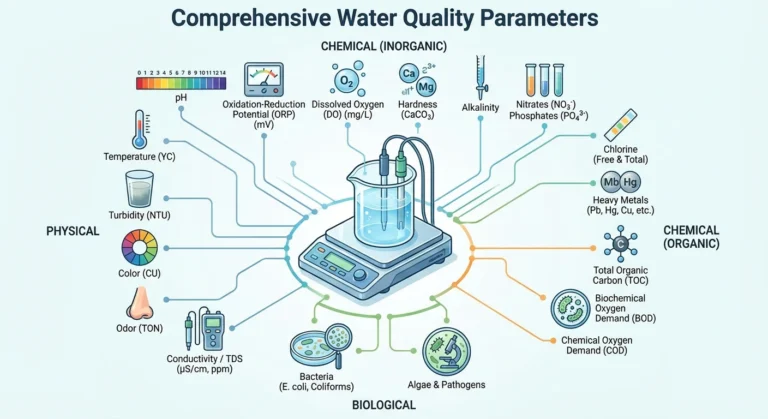
Understanding water quality is key to safe drinking water. The mineral content and pH of a seemingly pure cup of water cannot be seen. Professional water quality analysis devices turn “safety” into precise data. From laboratory analysis to online real-time monitoring, we help you understand every drop of water and make the healthiest decisions.
Request A Quote
More Resources
-
The 10 Best Digital carbon monoxide detectors 2026: An Industrial Guide
Carbon monoxide (CO) remains one of the most hazardous invisible threats in industrial, commercial, and residential environments. Colorless, odorless, and tasteless, it requires…
-
Can gas detectors detect multiple gases simultaneously?
In the complex and often hazardous world of industrial manufacturing, petrochemical processing, and confined space operations, ensuring the safety of personnel is the…
-
Surface Water vs Groundwater: A Comprehensive Guide to Water Quality
In the domains of environmental engineering and industrial water management, knowing the basic differences between surface water vs groundwater is not just an academic…
-
What is the pH of Reverse Osmosis Water? The Complete Science Guide (2026)
If you have ever been curious about ‘what is the pH of reverse osmosis water?’, you are not alone. This is one of…
-
The Top 8 Portable CO Detector for Car Use
Introduction: The Silent Threat in Automotive Cabins Carbon monoxide (CO) is a colorless, odorless, and highly toxic gas generated by the incomplete combustion…
-
6 Best Mass Flow Controller for Liquids: An Expert Technology Guide
In modern industrial automation, pharmaceutical manufacturing, and semiconductor fabrication, the precise control of fluid dynamics is a fundamental requirement. Relying on outdated volumetric…
.png)