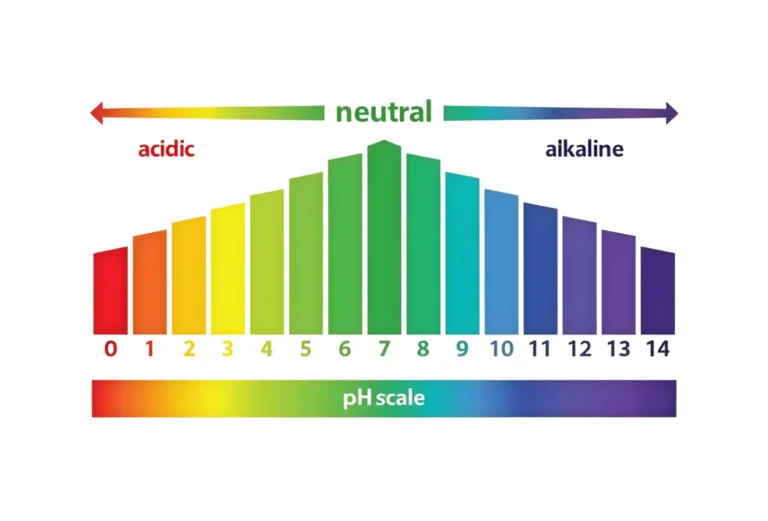
A lot of people think that distilled water is the purest since it has a pH of 7.0, which is perfect and neutral. If you were to test a bottle of distilled water that has just been opened, though, you might get a surprise result: a reading that is somewhat acidic, usually between 5.5 and 5.8.
In this article, we’ll talk about the chemistry behind why distilled water’s pH changes as soon as it reaches the air, why this is important for your pool or lab, and how to deal with it.

What is the ph of distilled water?
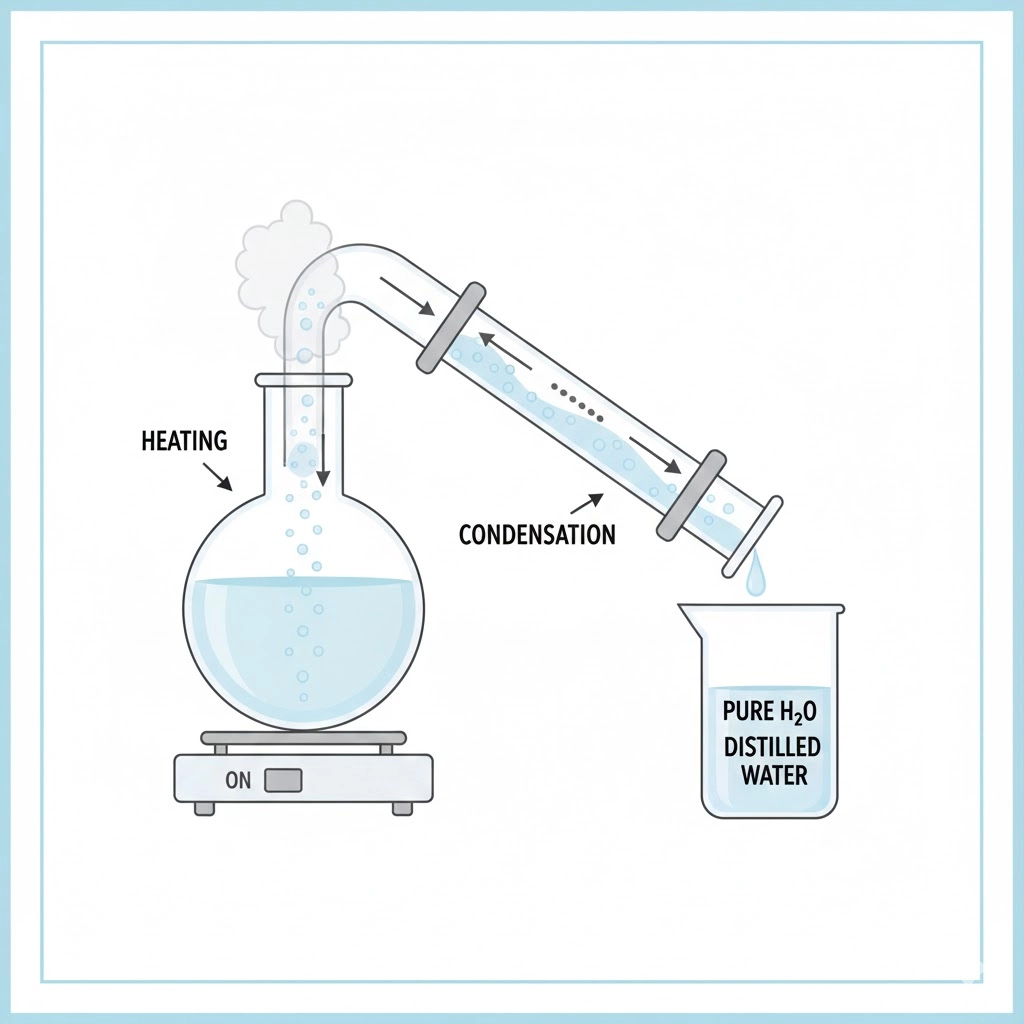
What is distilled water?
To manufacture distilled water, you heat it up, let it evaporate, let it cool down, and then collect it. The aim is to leverage the fact that water boils at 100 °C, which is different from the boiling points of other impurities like minerals and organic molecules, to turn it into steam. A condenser tube cools this steam down into liquid water, removing any pollutants that don’t evaporate.
- Key characteristics: extremely high purity, extremely low conductivity (almost non-conductive), and a pH value close to neutral (about 6.5-7.0).
- Purity classification: The higher the number of distillations, the higher the purity. It is divided into primary distilled water, secondary distilled water (re distilled water), and multiple distilled water.
The ph of distilled water
The perfect pH level for pure distilled water is 7, which is neutral. But in real life, it normally has modest acidity (pH 5.6–7.0) since it comes into touch with air.
The pH scale measures the concentration of hydrogen ions (H⁺) in water.However, pH measurement requires ionic conductivity to be stable and repeatable.
Distilled water:
- Has extremely low conductivity
- Responds instantly to contamination
- Changes pH easily
This makes its pH theoretically neutral, but practically unstable.

Can the pH of Distilled Water Change?
Yes — and very easily.
Because distilled water lacks buffering ions, even small environmental influences can cause measurable pH shifts within minutes.
What Makes the pH of Distilled Water Change?
- Taking in Carbon Dioxide
- When CO₂ dissolves, it undergoes a reaction: CO₂ + H₂O → H₂CO₃ This is the secret behind the acidity.
- Containers for storage
- Plastic, glass, or metal containers can leak small amounts of chemicals that change the pH.
- Temperature and Pressure
- Changes in temperature change the equilibrium of water’s dissociation, which changes the pH a little bit.

Why is the pH of distilled water not equal to 7?
Scientific Reality: “Buffering Vacuum”
Ideally, pure H₂O at 25C has a pH of 7.0 due to its perfect equilibrium of H⁺ and OH⁻ ions. However, distilled water is chemically “hungry.” Total Alkalinity is absent because calcium and magnesium are removed.
Water chemistry uses alkalinity as a pH “buffer” or shield.Distilled water has no atmospheric protection without this screen. The water absorbs CO₂ immediately after opening the container.
Absorption causes a chemical reaction:
CO₂ + H₂O → H₂CO₃ (Carbonic Acid)
This weak acid produces hydrogen ions, lowering pH from 7.0 to 5.8 in minutes. This is extreme purity influenced by the environment, not impurity.
How to Measure the pH of Distilled Water (And Why Most People Fail)
Measurement of clean water is notoriously difficult. If your readings drift or are inconsistent, you may be using the wrong equipment.
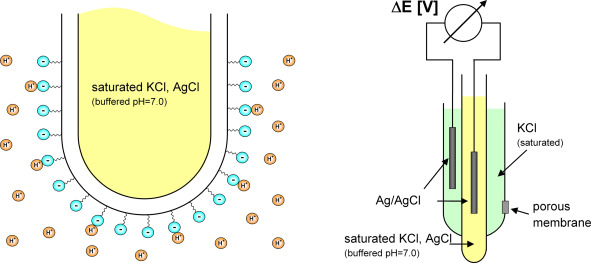
The Issue: The “Ghost” Reading Standard pH electrodes form an electrical circuit with a continuous ion flow. The liquid junction of a typical probe struggles to produce a steady potential in distilled water due to its low conductivity. Result: “ghost readings” that drift indefinitely.
Myth: pH Strips Never use pH strips for distilled water. Chemical dyes on the strip change the pH of the water droplet you’re analyzing because pure water has no buffering capacity. The strip measures its reaction to water, not the water.
The Sino-Inst Solution: Building for Low Conductivity You need a system that works well in high-impedance conditions to acquire a reading that is accurate in a lab. Our pH meters and online sensors at Sino-Inst have:
- Specialized Reference Systems: These have high-outflow liquid junctions that keep the electrical channel stable in water that doesn’t conduct electricity.
- High-Impedance Glass Bulbs: Made to work quickly in ultrapure and deionized process water.
- Shielded Electronics: This stops static from getting in the way, which is typical when evaluating very pure fluids.
💡 Pro Tip: The KCl Trace Method Add a single, small crystal of pure Potassium Chloride (KCl) to your 100ml sample if you are having trouble with a reading that keeps changing in the lab. This adds precisely the right amount of ions to make the electrode conductive without changing the pH balance too much. Your meter should stabilize right away.
Distilled Water vs Purified Water
| Parameter | Distilled Water | Purified Water |
|---|---|---|
| Minerals | Almost none | Very low |
| Conductivity | Extremely low | Low |
| pH Stability | Poor | Better |
| Typical Use | Lab, industry | Drinking, domestic |

The function of distilled water and “Can it be drunk
- Regarding applications: Distilled water is widely used in CPAP machines, automotive batteries, laboratory experiments, and steam irons.
- Regarding drinking: Distilled water is safe to drink. Although it lacks natural minerals and is slightly acidic, its acidity is much lower than coffee or soda water. Saliva and the gastric buffering system in the body can quickly neutralize this modest acidity.
Conclusion: Mastering the Science of Distilled Water pH
Distilled water reacts with chemicals in a very different way because it is so pure. Though the pH value should be 7.0, its “slight acidity” proves that it is pure.
Summary of the main points:
- Because pure water doesn’t have any minerals in it, it is very sensitive to changes in its environment.
- The pH level drops to 5.5 to 5.8 because the plant takes in CO2 from the air and changes it into carbonic acid.
- Measurement rules: Get rid of the pH test paper. If you want to measure pure water consistently, you must use either the trace KCl method or a high-precision electrode.
- Usefulness: Understanding this acid-base change is important for keeping precise tools in good shape, running lab tests, and keeping pool water clean.
FAQ
Related Products
pH/ORP Controllers
The “slight acidity” of distilled water is a great example of chemistry in action. But in factories and labs, an incorrect reading can cause expensive mistakes in making products or keeping equipment in good shape.
We at Sino-Inst know that pure water needs pure accuracy. Our specialized pH controllers and sensors make it easy to monitor low-conductivity levels, providing you solid, accurate data you can trust.
Are you ready to improve your accuracy? [👉 Look through our pH controllers] or get a quote from us immediately.
Request a Quote
More Resources
-
How Do You Test for Helium?
Helium is a unique element. It is the second lightest element in the universe, chemically inert, non-flammable, and possesses the smallest atomic size…
-
How Do I Get My Carbon Monoxide Detector to Stop Chirping? (2026 Guide)
The piercing sound of a carbon monoxide (CO) detector chirping is one of the most stressful noises a homeowner can experience. It often…
-
Choosing a pH Meter for Water:The Professional Guide to(2026)
A pH meter for water is the pulse of chemical equilibrium in water treatment and industrial processing, far more than just a digital…
-
9 Best Personal Gas Monitoring Devices 2026
In the industrial world, safety is not a luxury; it is a prerequisite for operation. As we move into 2026, the technology driving…
-
What Is Dissolved Oxygen in Water? The Ultimate Guide to Monitoring(2026)
When we talk about water quality, we often focus on what we can see, like clarity, or what we can taste, like salt….
-
Mastering Safety: How to Check Gas Level in Confined Space?
The Critical Importance of Atmospheric Testing Working in industrial environments often requires entering areas that are not designed for continuous occupancy. These locations,…
.png)