Test water for ph is the most critical defense against system failure in water treatment and industrial processing. It is the pulse of chemical equilibrium—far more than just a digital reading. Whether you are managing urban sewage treatment facilities, high-tech hydroponic farms, or sensitive pharmaceutical laboratories, the decision to test water for ph accurately is the essential first step toward achieving operational excellence.

What is the pH Scale?
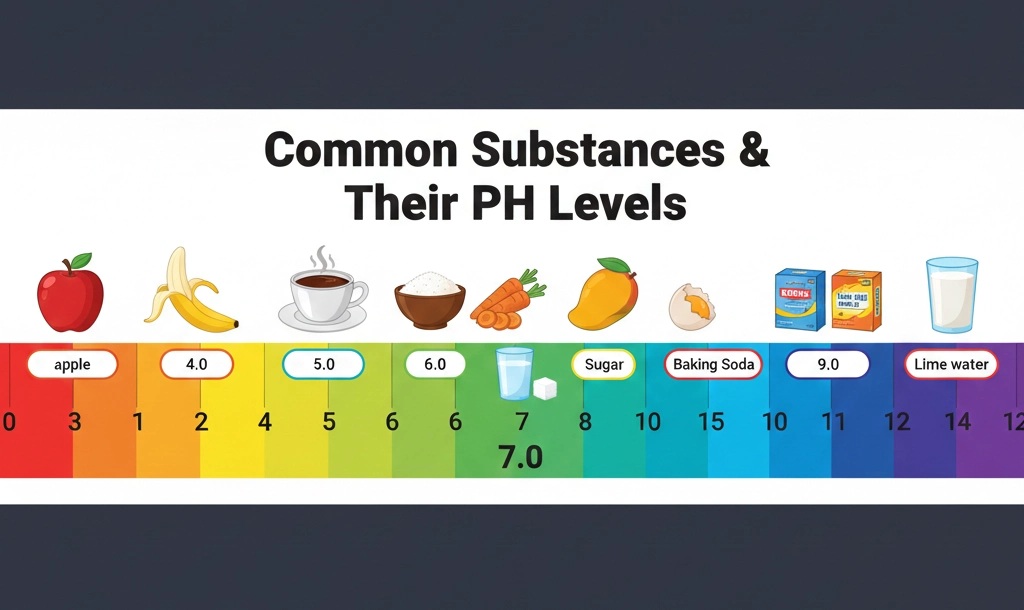
Before diving into testing, we must define what we are measuring.The pH scale (Potential of Hydrogen) ranges from 0 to 14. It measures the concentration of hydrogen ions in a solution:
- pH < 7: Acidic (High concentration of H⁺)
- pH = 7: Neutral (Pure water)
- pH > 7: Alkaline/Basic (Low concentration of H⁺)
The 10x Factor: The scale is logarithmic. This means pH 5 is 10 times more acidic than pH 6, and 100 times more acidic than pH 7. Small numerical shifts represent massive chemical changes.
How to test for pH in water?
Depending on your accuracy needs, there are three primary ways to measure:
- Chemical Indicators (Test Strips): Fast and cheap, but rely on visual color matching. Unsuitable for industrial precision.
- Portable Digital Meters: The “Scout” of the field. Battery-powered devices that provide high accuracy for spot-checks.
- Online pH Controllers: The “Brain” of the factory. Fixed sensors that provide 24/7 monitoring and can automatically control dosing pumps to maintain balance.
💡 Pro Insight: To avoid the “Fatal Mistakes” mentioned below, you must first ensure you are using the right hardware for your specific environment. Accuracy starts with the tool itself. For a deep dive into choosing the right equipment, check out our [Professional Guide to Choosing a pH Meter for Water (2026)].
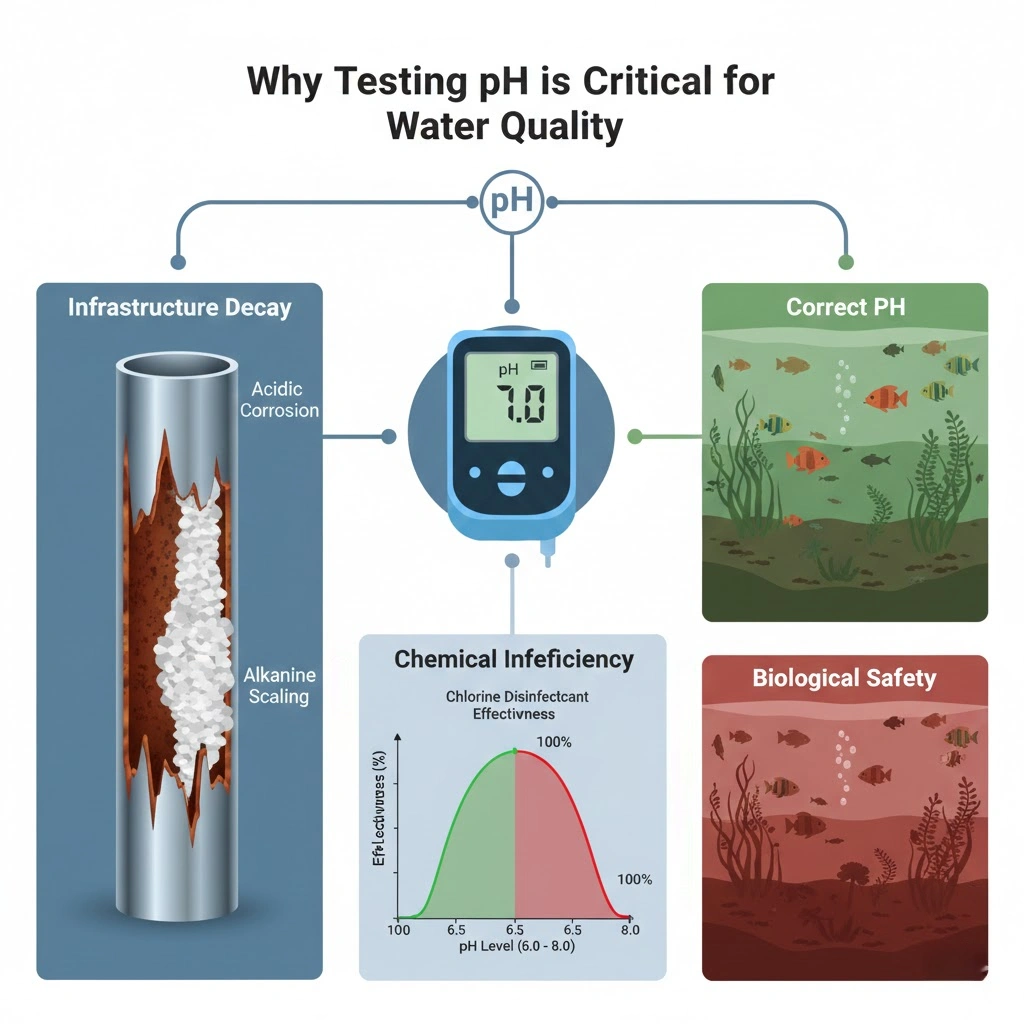
Why Testing pH is Critical for Water Quality
There is a direct link between pH and the quality of water. If you don’t check the pH of water, you could:
- Infrastructure Decay: Acidic water eats away at metal pipes (corrosion), and alkaline water builds up minerals in systems (scaling).
- Chemical Inefficiency: If the pH in water is not between 6.5 and 7.5, disinfectants like chlorine can lose up to 90% of their power.
- Biological Safety: If the pH of water is wrong in wastewater or aquaculture, it can destroy the good bacteria or aquatic life that is needed for the process.
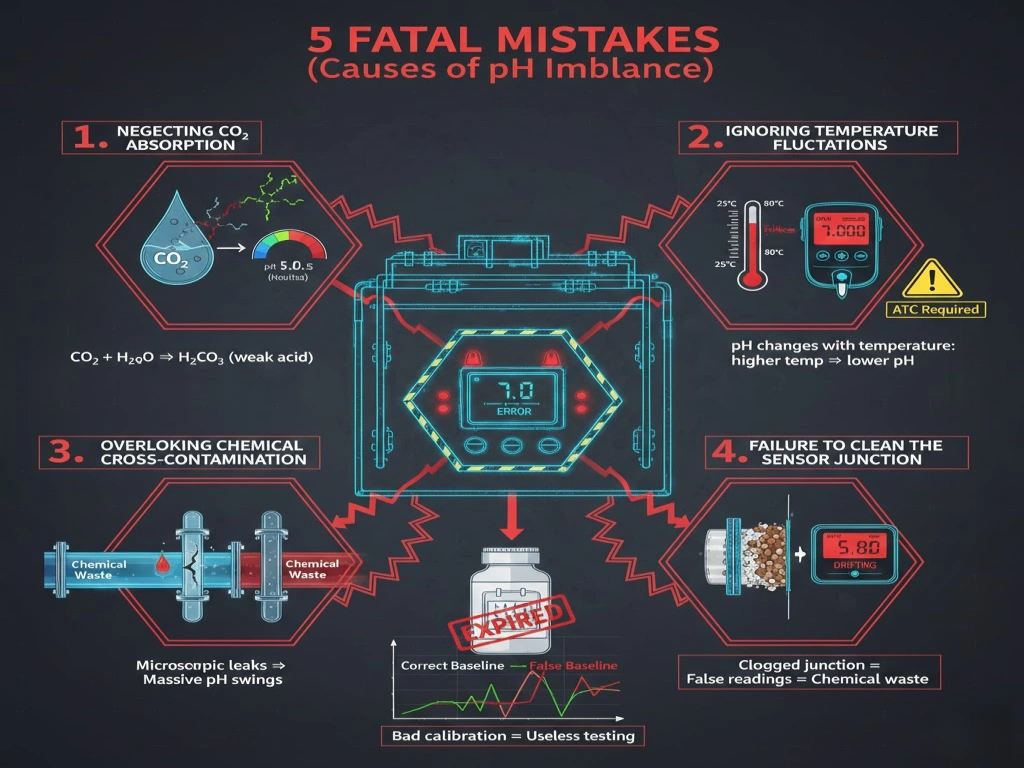
5 Fatal Mistakes (Causes of pH Imbalance)
Why does pH of water fluctuate? Most operators fail because they overlook these five causes:
Neglecting CO₂ Absorption
When water is exposed to air, it absorbs carbon dioxide, forming weak carbonic acid. This is the #1 reason why “pure” water often tests as slightly acidic.
Ignoring Temperature Fluctuations
pH in wtaeis temperature-dependent. As water heats up, molecules vibrate faster, changing the ion activity. Testing without Automatic Temperature Compensation (ATC) is a fatal data error.
Overlooking Chemical Cross-Contamination
In industrial lines, even a microscopic leak from a cleaning cycle or an upstream process can swing the pH of thousands of gallons of water.
Failure to Clean the Sensor Junction
The most common “mistake” isn’t the water—it’s the tool. A clogged reference junction on your meter will cause the reading to “drift,” leading you to add chemicals to water that was already balanced.
Using Expired or Contaminated Buffers
Testing is only as good as your calibration. Using a buffer solution that has been sitting open for months will give you a “false” baseline, making your entire testing process useless.
Avoiding these errors is crucial for accurate ph water testing in industrial environments.
Troubleshooting table
| Symptom | Potential Cause | Solution |
| Reading Drifts | Clogged reference junction | Clean with 0.1M HCl or pepsin |
| Slow Response | Dehydrated glass bulb | Soak in pH 4 buffer or KCl for 4 hours |
| Erratic Values | Low conductivity or ground loop | Use a low-impedance sensor/ground the tank |
Testing pH Across Different Applications
Depending on your industry, the way you test water for ph will vary significantly:
- Wastewater Treatment: You must test water for ph to prevent the destruction of biological flora. Sensors must be rugged to resist oil and scale.
- Hydroponics: Constant monitoring is required because a small drift can prevent plants from absorbing nutrients (nutrient lockout).
- Boiler Water: High-purity water requires special electrodes to test water for ph because low conductivity can cause massive signal noise.
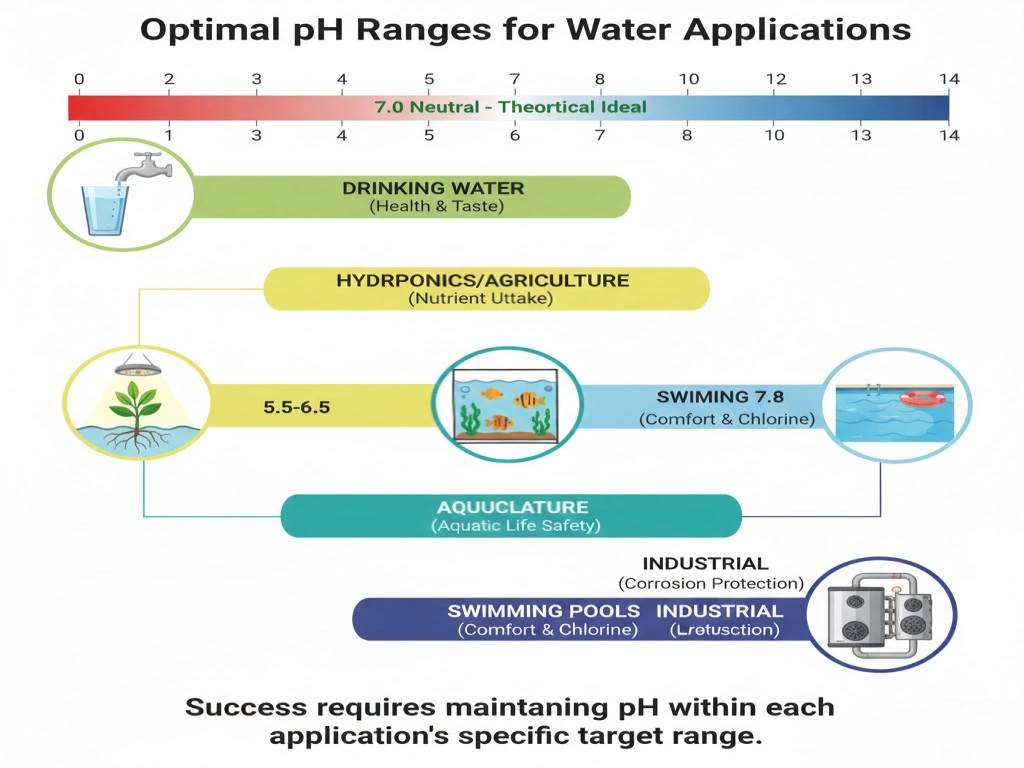
What’s the best pH for water?
The optimal pH level depends on how you plan to use it. Common ranges in a number of areas:
- Water that is 6.5 to 8.5 parts per million meets most health regulations,keeps metal from leaching from pipes,and tastes fantastic.
- Industrial boilers and cooling towers:8.0-10.0 generate a passivation layer that protects against acidic corrosion in a slightly alkaline atmosphere.
- Aquaculture:7.0-8.2 mimics the native habitat of most aquatic organisms to avoid”pH shock.”
- Hydroponic and agricultural:The ideal acidity level for plant roots to take up minerals is between 5.5 and 6.5.
- For comfort and to make sure chlorine works as a bactericide,the swimming pool should be between 7.2 and 7.8.
- The main points is that pH 7.0(neutral) is best, but to be successful,you need to make sure that the pH value stays within the “target range”that the application demands.
Pro Tip: Selection & Calibration
To avoid these mistakes, you need the right hardware.
- Selection: For 24/7 stability, choose an Online pH Controller like the Sino-Inst A11PR.
- The 3-S Calibration Rule: Store in KCl solution, Stop clogging with monthly cleanings, and Schedule calibration every 14 days.
Compliance Matters: In industries like pharmaceuticals and food processing, ph test for water must comply with standards such as EPA 150.1 or ISO 10523. Using a certified online controller ensures your data logs are audit-ready, shielding your facility from legal liabilities.
Why Calibration is the Key to Testing When you test water for ph, your data is only as reliable as your last calibration. Beyond the 3-S rule, professional operators look at the Slope (S) and Offset (E0).
- The Slope: Should be between 95% and 102%. If it falls below 85%, your sensor is end-of-life.
- The Offset: Should be within ±30mV at pH 7.00. These metrics ensure that every time you test water for ph, you are getting the scientific truth, not a drifting estimate.
Because this step is so vital to your system’s longevity, we have dedicated a full technical breakdown to it. Learn the industry-standard 3-S Rule in our featured post: [Calibration of pH Meter for Water].
FAQ
Related Products
Stop guessing and start taking charge.
To be precise, you need the correct tools and a lot of practice. Don’t let these 5 fatal mistakes ruin your infrastructure or product quality. At Sino-Inst, we don’t just sell tools; we provide the professional-grade online controllers and sensors needed to turn your pH testing into an exact science.
Ready to upgrade your accuracy? [Get a Free Technical Consultation & Quote Today] and let our experts help you find the perfect pH solution for your specific application.
Request A Quote
More Resources
-
How to Get Rid of Chlorine in Water — The Complete Science-Backed Guide (2026)
If you’re searching for how to get rid of chlorine in water, you probably want better‑tasting tap water, healthier skin, or safe water…
-
Is It Safe to Drink Purified Water? Practical Guide to Making a Healthy Choice.
Is it safe to drink purified water? “Is the most crucial question for health conscious consumers when choosing bottled water or home water…
-
5 Kinds of Detector Detects Gas Leaks: Expert Selection Guide
In industrial safety, the margin for error is non-existent. Whether managing a petrochemical refinery, an agricultural greenhouse, or a manufacturing plant, the ability…
-
Total Chlorine vs Free Chlorine: The Complete Industrial Monitoring Guide (2026)
Introduction:Why the Chlorine”Guess”Costs You Money? In water treatment,precision isn’t just a goal;it’s a safety requirement.Many operators ask:”Is total chlorine vs free chlorine same?“The…
-
5 Expert Tips to Put an Explosive Gas Detector for Maximum Safety
Safety is not a variable; it is a constant requirement in industrial process control. When dealing with combustible gases, the difference between a…
-
9 Best VOC Detectors for 3D Printing Safety (2026 Guide)
As 3D printing technology moves from industrial factories to home workshops and small businesses, air quality safety has become a paramount concern. Whether…
.png)