Why should you care about pH sensor calibration more than you think? Whether you are managing wastewater treatment, chemical processing, or food manufacturing, a pH sensor is only as accurate as its last calibration.
Over time, all pH electrodes suffer from signal drift caused by aging, fouling, and temperature shifts. A poorly calibrated sensor leads to chemical overdosing, failed quality control, and increased operating costs.
In this guide, we provide a practical, field-oriented tutorial on how to calibrate your sensors, avoid common mistakes, and recognize when it’s time to stop calibrating and start replacing.

By the end of this guide, you will understand:
- How pH calibration actually works
- Which calibration method fits your application
- How to recognize when a sensor should be replaced instead of recalibrated
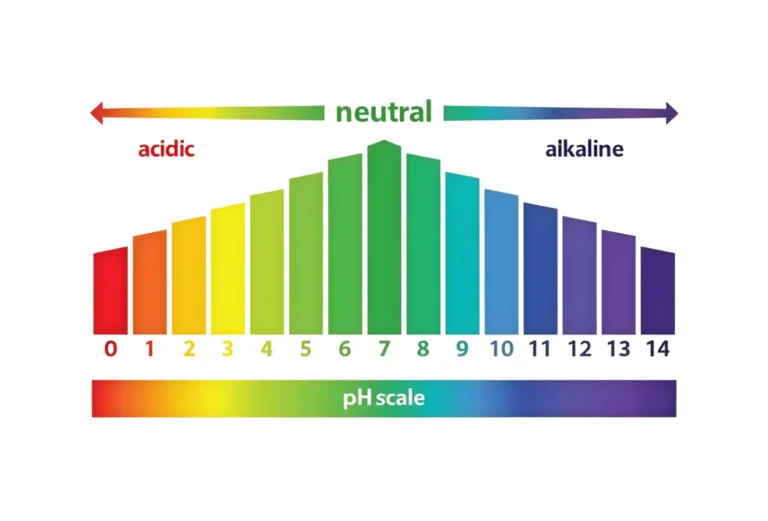
What Is pH Sensor Calibration?
The process of calibrating a pH sensor involves changing the sensor’s output such that it matches recognized reference values given by standard buffer solutions.
To put it simply:
- The buffer solution has a pH that is known.
- The output from the sensor is changed to match that number.
- This corrects signal drift caused by electrode aging and contamination.
Most modern pH meters and controllers support automatic calibration, but understanding the underlying principles helps with troubleshooting later.

Risks of Skipping pH Sensor Calibration
From an operational standpoint, uncalibrated pH sensors introduce measurable risks:
- Chemical over-dosing or under-dosing
- Process instability and control loop oscillation
- Failed quality control or regulatory non-compliance
- Increased chemical consumption and operating costs
In wastewater treatment, chemical processing, and food production, pH sensor calibration directly affects safety, cost, and product quality.
Why Do pH Sensors Lose Accuracy? (The Science of Drift)
Even the highest quality industrial pH sensors are consumables. Understanding why they drift helps you set a better maintenance schedule:
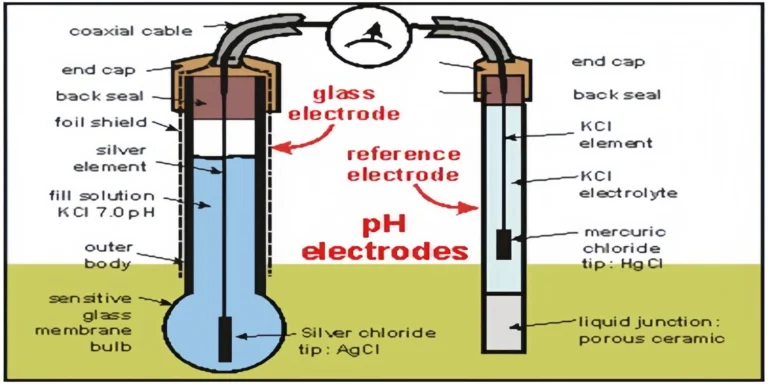
- Glass Membrane Aging: The sensitive glass bulb loses its ability to exchange hydrogen ions over time, leading to a “low slope” (reduced sensitivity).
- Reference Junction Clogging: In wastewater or high-solids applications, the junction can get blocked by oils or biofilms, causing “noisy” or jumping readings.
- Temperature Variation: pH is a temperature-dependent measurement. Without Automatic Temperature Compensation (ATC), your readings will never be truly accurate.
👉 Calibration cannot repair physical damage, it only helps detect when the sensor can no longer be used accurately.
Sino-Inst’s expert opinions
We at Sino-Inst have found that a lot of calibration problems are not caused by what the user does, but by choosing the wrong sensor.
For instance, typical ceramic junction electrodes often break quickly in wastewater or conditions with a lot of solids, which causes calibration drift to happen commonly. That’s why Sino Inst makes pH sensors that are specifically made for certain applications, such PTFE junction and flat-surface electrodes, that work best in wastewater, high-solids, and fouling-prone conditions.
pH Sensor Calibration Principles
The Nernst equation describes a straight line between pH value and electrode voltage. This is how pH sensors work.
The theoretical slope at 25°C is:
- 59.16 mV for every pH unit
When calibrating, the instrument changes:
- Offset (the zero point is at pH 7)
- Slope (how sensitive it is across the pH range)I
If the slope drops below an acceptable level, successful calibration may no longer be possible. This is often the first clue that something has to be replaced.

Calibration Methods Explained (With Comparison Table)
1. Mid-Point Calibration
Uses one buffer, usually pH 7.00.
| Feature | Description |
|---|---|
| Accuracy | Low |
| Corrects offset | ✅ |
| Corrects slope | ❌ |
| Typical use | Quick checks only |
📌 Not recommended for industrial control.
2. Two-Point Calibration (Most Common)
Uses:
- pH 7.00
- pH 4.00 or pH 10.00
| Feature | Description |
|---|---|
| Accuracy | Good |
| Corrects offset | ✅ |
| Corrects slope | ✅ |
| Typical use | Industrial & lab applications |
This is the standard calibration method for most users.
Most Sino-Inst industrial pH controllers support automatic two-point calibration with real-time slope and offset display, allowing users to quickly evaluate sensor health during calibration.
3. Three-Point Calibration
Uses pH 4.00, 7.00, and 10.00.
| Feature | Description |
|---|---|
| Accuracy | High |
| pH range | Wide |
| Complexity | Higher |
| Typical use | High-precision laboratories |
Step-by-Step pH Sensor Calibration Procedure
To make sure your sensor is correct, not just “calibrated,” do these things:
- First, clean the electrode by running it under deionized water. If it was in oily water, mix 5% HCl with water or use a light detergent first.
- For a lint-free dry, use a blot, not a rub. When you rub, you make static energy, which makes the reading move around for minutes.
- In order to set the Zero Point (Offset), you should always begin with a pH level of 7.00.
- Being stable is very important. Don’t confirm the calibration point until the mV number stays the same for at least 30 seconds.
The Slope Test: Check your controller’s Slope percentage after the second point (pH 4 or 10).
Troubleshooting: Why Your Calibration Fails
If your calibration keeps failing, look for these “hidden” culprits:
- The 85% Rule: If your slope falls below 85% (approx. 50 mV/pH), the glass membrane is chemically exhausted. Stop calibrating—it’s time to replace the sensor.
- The Offset Error: If your pH 7.00 reading shows more than ±30 mV, your reference junction is likely contaminated or the electrolyte is depleted.
- Expired Buffers: pH 10.01 buffer is notoriously unstable as it absorbs $CO_2$ from the air. Always use fresh, sealed buffer solutions.

Field Experience from Sino-Inst
In industrial environments, repeated calibration failure is often the first warning sign of sensor end-of-life.
Based on sino-inst field data, when a pH electrode cannot maintain a slope above 85% after proper cleaning, continued calibration no longer improves accuracy and replacement becomes the most cost-effective solution.
Automatic Precision Takes the Place of Manual Hassle
In factory settings with a lot of use, manual calibration becomes a labor-intensive bottleneck. This is the reason why a lot of places are switching to Sino-Inst Digital pH Systems.
The calibration data for our digital pH sensors is stored right in the sensor head. This lets you:
- Plug-and-Play: Set it up in the lab and use it right away in the field after swapping it out.
- Health Tracking: Sensor slope and glass resistance are checked in real time.
- Longer Lifespan: PTFE junctions and flat-surface electrodes that are specially made for the harshest wastewater conditions.
Conclusion: pH Sensor Calibration Is Required
You need pH sensor calibration because:
A stable calibration means:
- Reliable measurements
- Lower chemical consumption
- Better process control
Regular pH sensor calibration is necessary for accurate results, robust operations, and lower operating costs.
If calibration is difficult or impossible, replacement is usually cheaper than repair.
FAQ
Related Products
Glass Sensor
Plastic-Shell Sensor
Looking for a Reliable Replacement?
Sino-Inst provides a full range of industrial pH sensors and controllers, designed for:
- Wastewater treatment
- Chemical processing
- High-temperature and high-solid applications
If you are unsure which pH sensor fits your process, the Sino-Inst technical team can help you select the right model based on your operating conditions.
Request a Quote
More Resources
-
How Do You Test for Helium?
Helium is a unique element. It is the second lightest element in the universe, chemically inert, non-flammable, and possesses the smallest atomic size…
-
How Do I Get My Carbon Monoxide Detector to Stop Chirping? (2026 Guide)
The piercing sound of a carbon monoxide (CO) detector chirping is one of the most stressful noises a homeowner can experience. It often…
-
Choosing a pH Meter for Water:The Professional Guide to(2026)
A pH meter for water is the pulse of chemical equilibrium in water treatment and industrial processing, far more than just a digital…
-
9 Best Personal Gas Monitoring Devices 2026
In the industrial world, safety is not a luxury; it is a prerequisite for operation. As we move into 2026, the technology driving…
-
What Is Dissolved Oxygen in Water? The Ultimate Guide to Monitoring(2026)
When we talk about water quality, we often focus on what we can see, like clarity, or what we can taste, like salt….
-
Mastering Safety: How to Check Gas Level in Confined Space?
The Critical Importance of Atmospheric Testing Working in industrial environments often requires entering areas that are not designed for continuous occupancy. These locations,…
.png)